four microcentrifuge tubes shown represent a serial dilution series from|serial dilution flashcards : supplier The four microcentrifuge tubes shown represent a serial dilution series from the original sample. What is the total dilution of the fourth microcentrifuge tube in this serial dilution . webIf you want to access your files in Koofr with the file manager on your computer which has a built-in DAV connection, follow the steps below: Open your Account Settings in Koofr App and select Preferences. Click on Password in the menu on the left. Scroll to the Generate new password textbox, type in the password name and click Generate.
{plog:ftitle_list}
WEBPub: 30 Jul 2023 22:00 UTC Views: 313. new·what·how·langs·contacts·what·how·langs·contacts
The four microcentrifuge tubes shown represent a serial dilution series from the original sample. What is the total dilution of the fourth microcentrifuge tube in this serial dilution .
The four microcentrifuge tubes shown represent a serial dilution series from the original sample. What is the total dilution of the fourth microcentrifuge tube in this serial dilution .CFU/ml in the original sample (don't forget units!): 7.5 x 10^7 CFU/ml. Calculate the following. An example for how to type exponents into Canvas: 10^-1 (use the carrot: shift-6) Individual dilution factors at each tube. tube 1:b. The pipetting error can be minimized by using larger volumes. That's also the reason why you're prepared the dilutions in the micro centrifuge tubes and not directly in the microplate .
A serial dilution is any dilution in which the concentration decreases by the same factor in each successive step. In serial dilutions, you multiply the dilution factors for each step. .
Create a series of solutions of decreasing concentrations via serial dilutions. Use the spectrophotometer to measure the absorbance of a solution. Use excel and make a standard curve and use the R2 value to evaluate the .To perform a viable cell count, a sample of bacterial cells is first diluted through a series of dilutions. (A series of dilutions is required because the original concentration of cells is . The objective of the serial dilution method is to estimate the concentration (number of organisms, bacteria, viruses, or colonies) of an unknown sample by the enumeration of the number of colonies cultured from .
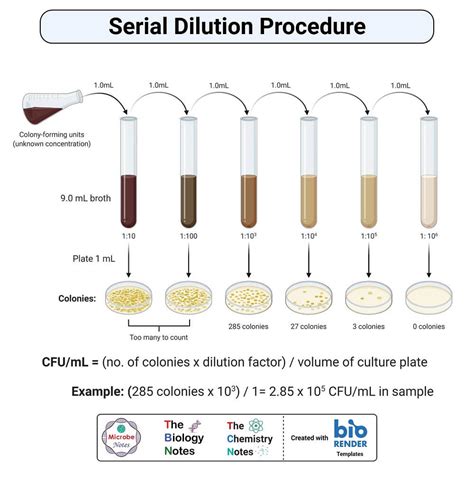
1. Plan a 1/100 dilution series so that each dilution has a total volume of 1 ml. The concentration of your final dilution should be 10-15 g/ml. Record your plan in your notebook. 2. Set up and . Upon completion of this lab, students will be able to: Create a series of solutions of decreasing concentrations via serial dilutions. Measure the absorbance of solutions with a microplate reader. Generate standard curves in .
Question: Examine the picture that represents the serial dilution of a bacterial culture, then answer the questions that follow. An illustration shows the process of serial dilution. The illustration consists of 4 test tubes and 2 Petri plates. 4 test tubes were labeled as the original sample is diluted several times.
Study with Quizlet and memorize flashcards containing terms like You found 67 colonies on the ground beef plate with the 10-2 dilution. What is the concentration of bacteria per milliliter of beef? 6.7 × 103 CFU/ml 6.7 × 104 CFU/ml 6.7 × 102 CFU/ml 67, Which of the following is indicative of the presence of E. coli? colonies on EMB agar with a metallic sheen colonies on .Study with Quizlet and memorize flashcards containing terms like With respect to the image of a microscope specimen, refraction of light by the objective lens enhances __________., In a compound light microscope, what is the function of the condenser?, You should begin viewing a specimen with what objective lens? and more. Procedure of Serial Dilution. The following is the procedure for a ten-fold dilution of a sample to a dilution factor of 10-6:. The sample/culture is taken in a test tube and six test tubes, each with 9 ml of sterile diluents, which can either . Preparation of diluent Prepare six test tubes that can store 20 mL or more in a rack and label them T1-T6. Each tube is consistent with the dilution factor it corresponds to (i.e., T3= 1×10 3 OR 0.001th of stock concentration).; Pipet 9 mL of sterile water, saline, or broth into each of the 10 test tubes.
Question: Procedure: Step 1: Obtain metal sample in test tube. First add a test tube to the Lab. Select the test tube and add 100 gm of Iron Shot (Fe). Add a thermometer to the test tube so that you can record the initial temperature of the Fe. Step 2: Prepare hot water bath and add test tube with metal. Obtain a 250 ml beaker.
serial dilution test
MCCC BIO201 Microbiology Laboratory Instructional Videos
A serial dilution is a step-wise series of dilutions, where the dilution factor stays the same for each step. . inhibitory concentration of 20 µg/ml from the 10-fold serial dilution experiment and 12.5 µg/ml from the 2-fold serial dilution method, as shown in this table: Figure 4: Serial dilution chart comparing the compound concentrations .Study with Quizlet and memorize flashcards containing terms like What is the formula for calculating CFU/mL?, You perform a serial dilution and find 51 colonies on a spread plate from a tube with a 10^-5 dilution factor. If you plated 0.1 mL, what is the concentration of viable cells in the original sample?, You count 162 colonies after you plate 0.1 mL from a tube with a .2 into one microcentrifuge tube for each dilution that will be made. (For example, if making dilutions for a titer, prepare 10 tubes with 90 μL Phage Buffer w/ 1mM CaCl 2.) 2. Label the tubes with the dilution number. 3. Remove 10 μL of the “neat” (undiluted) phage sample and add it to the first dilution tube (labeled 10–1). Vortex for .
http://technologyinscience.blogspot.com/2014/08/serial-dilution-of-bacterial-culture.htmlSerial Dilution is a series of sequential dilution of a substance in.
Figure 1. A ten-fold serial dilution, which can also be called a 1:10 dilution, or a series with dilution factor of 10. To determine the concentration at each step of the series, you divide the previous concentration by the dilution factor. *Dilution tubes begin with 9-mL. 1-mL is added and mixed, then 1-mL is transferred to the next tube.Serum (preferred): trace element free plain royal blue top tube. Plasma: heparin or EDTA. Do not use gel barrier tube. Avoid all sources of external contamination. Ammonia (NH3) Plasma: EDTA (some use green no gel) Place immediately in an icy slurry. Centrifuge within 15 mins without removing stopper; separate plasma and freeze in plastic vial.Calculate the following. An example for how to type exponents into Canvas: 10^-1 (use the carrot: shift-6) Individual dilution factors at each tube tube 1: tube 2: tube 3: Individual dilution factors at each plate plate 1: plate 2: plate 3: Total dilution factors at each tube tube 1: tube 2: tube 3: Total dilution factors at each plate plate 1: plate 2: plate 3: CFU/ml in the original sample . In an experiment involving concentration curves, you can use a serial dilution to create a series of solutions with dilutions of 1, 1:10, 1:100, 1:1,000. Advertisement. Method . The final dilution factor of the fourth tube .
Introduction. A serial dilution is a series of dilutions made sequentially, using the same dilution factor for each step.The concentration factor is the initial volume divided by the final solution volume; the dilution .Whether you're preparing, transferring or storing samples, these multipurpose microcentrifuge tubes and caps can be used with confidence. Choose from a selection of tubes and caps that will suit your application. . Serial Dilution. .Whether you're preparing, transferring or storing samples, these multipurpose microcentrifuge tubes and caps can be used with confidence. Choose from a selection of tubes and caps that will suit your application. . Serial Dilution. Sample Preparation. Plate Transfer. Sample Preparation. Sample Storage. Centrifugation :
Simple step-by-step instructions to help you make dilutions and serial dilutions. Calculating dilution factors has never been easier! Jump to main content. Search. Search. Close. Resource Type: . A serial dilution is a stepwise series of dilutions. Each dilution in the series is used to make the next dilution until you have a whole set of .The beaker is labeled dilution 3. 4) 1.00 mL of the solution in the beaker labeled dilution 3 is transferred over to another beaker called dilution 4 and enough water is added so that the final volume is 5.00 mL and the concentration of the final dilution is 2.10 x 10-6 M. Solution: 1) from dilution 4 to dilution 3:The last portion of this exercise is to calculate the correct dilution factors for various serial dilutions. Macro Serial Dilution: 1. Obtain one 2 ml tube containing Malachite Green stain/dye, one flask with sterile DIW and four 16 mm tubes. 2. Label the Tubes 3. You will make a serial dilution with the dye. The final dilution should be 10-6 . Serial dilutions are a common practice in the natural sciences. Due to the period decrease in concentration, this method is very useful when performing many types of experiments, from chemistry to biology to medicine.You can plot the information they provide onto a graph to find the gradient and intercepts (or calculate them with our slope intercept form .
1. Plan a 1/100 dilution series so that each dilution has a total volume of 1 ml. The concentration of your final dilution should be 10-15 g/ml. Record your plan in your notebook. 2. Set up and label the microcentrifuge tubes needed for your dilution series and transfer the necessary amount of ultrapure water to each tube. 3.2. the microcentrifuge tube holds at most 1.5 ml. what is the most convenient volume to use for each dilution in the series?why? 3. using the least number of micorcentrifuge tubes, outline a serial dilution that results in an end dilution factor of 10^-7. 4. rethink the dilution series. you know that you will use the dilutions of 10^-5, 10^-6 .You are preparing a 4-tube series of a 5-fold serial dilution. If Tube #1 contains 500 µL of undiluted stock solution, then how much diluent should be added to Tubes #2 - #4? [2 pt; G3.3] 100 µL 250 µL 400 µL 500 µL. You are preparing a 4-tube series of a 5-fold serial dilution.Tube A shows the reddish-pink aqueous layer indicative of Indole production. 2. Positive for Indole Production 3. Kovacs Reagent 4. tryptophan 5. Pyruvate. 1. Of the 2 SIM tubes shown here, which one is positive for Indole production? (enter the letter of the correct tube) _____ 2. In Tube A, what does the pink layer on top of the SIM media .
We will show data from a water-based serial dilution set-up, isolating key parameters to demonstrate how they influence . performed directly or within a series of equal steps (Figure 1). The . We analyzed a 10-step, 2-fold serial dilution and a 4-step, 5-fold serial dilution at the LOD in 3 independent approaches (n=3). The
serial dilution micro lab 2
28 de abr. de 2023 · No vídeo, Kerolay Chaves colocou seu melhor pijama, bem cavado no bumbum, enquanto se joga na cama e leva os fãs à loucura. Atualmente, a produtora de conteúdo adulto conta com mais de 566 mil seguidores no Instagram. “Pulando na piscina, ou na cama? Que mergulho foi esse”, brincou um fã no campo de comentários.
four microcentrifuge tubes shown represent a serial dilution series from|serial dilution flashcards